Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
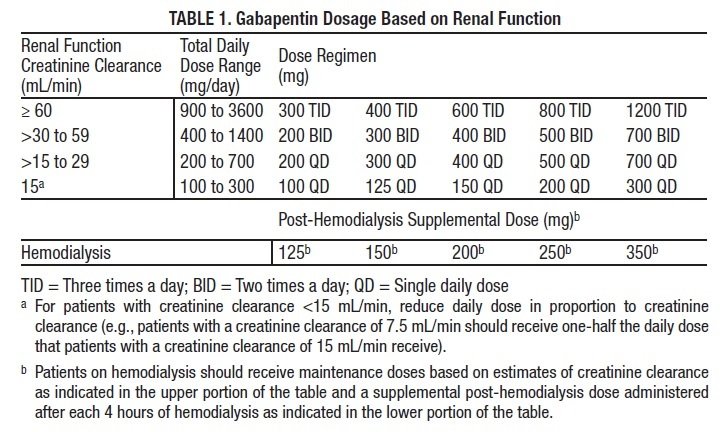 |  |
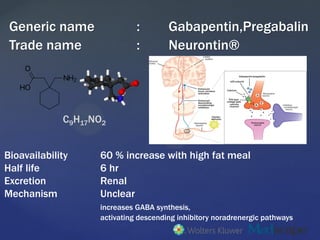
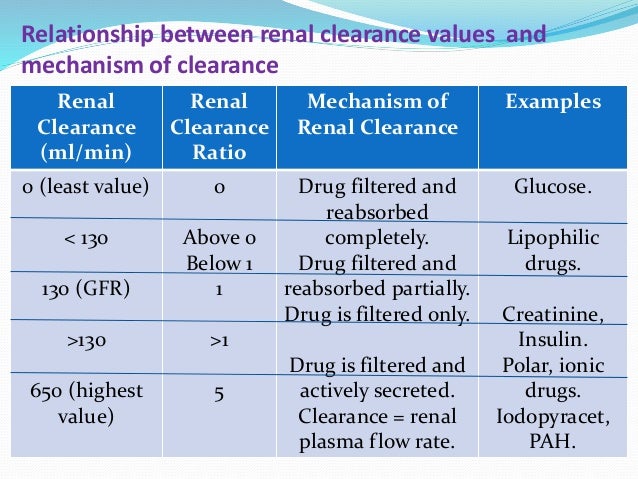
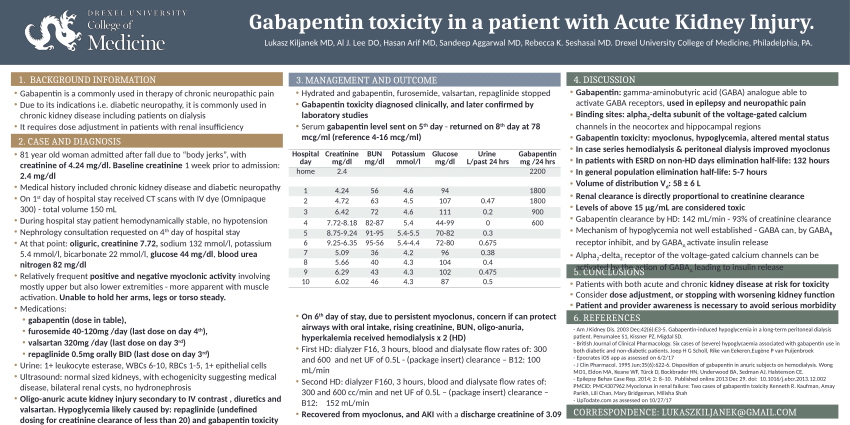
Background Gabapentin enacarbil, a transported acyloxyalkylcarbamate prodrug of gabapentin, provides predictable and dose-proportional gabapentin exposure (AUC). Gabapentin is cleared via renal excretion, and its elimination is proportional to creatinine clearance (CrCL); CrCL can, therefore, be used as a predictor of gabapentin renal clearance. Gabapentin clearance is directly proportional to creatinine clearance, and renal impairment reduces gabapentin excretion and increases plasma gabapentin concentrations in a linear fashion 8. The elimination half-life of gabapentin increases to 132 hours in patients on dialysis, compared to 5-9 hours in patients with normal renal function 8. The pharmacokinetic characteristics of gabapentin are described, and the interaction effects of concomitant antiseizure medications on gabapentin in terms of its pharmacokinetics and pharmacodynamics are highlighted. Accumulation can cause renal failure resulting in adverse effects. Formulations Gabapentin is available in two extended-release formulations in addition to the immediate release: a gastric retentive formulation (GBP-GR) and a gastro-retentive prodrug gabapentin enacarbil that are approved for the management of postherpetic neuralgia. Since gabapentin is almost exclusively eliminated by renal excretion, the larger treatment effect observed in patients greater than or equal to 75 years may be a consequence of increased gabapentin exposure for a given dose that results from an age-related decrease in renal function. Abstract Background: Gabapentin enacarbil, a transported acyloxyalkylcarbamate prodrug of gabapentin, provides predictable and dose-proportional gabapentin exposure (AUC). Gabapentin is cleared via renal excretion, and its elimination is proportional to creatinine clearance (CrCL); CrCL can, therefore, be used as a predictor of gabapentin renal clearance. Gabapentin produced from hydrolysis of Since gabapentin is almost exclusively eliminated by renal excretion, the larger treatment effect observed in patients ≥75 years may be a consequence of increased gabapentin exposure for a given dose that results from an age-related decrease in renal function. Since gabapentin is almost exclusively eliminated by renal excretion, the larger treatment effect observed in patients ≥75 years may be a consequence of increased gabapentin exposure for a given dose that results from an age-related decrease in renal function. Gabapentin is widely used in the management of pain. It is entirely excreted through the renal system so this needs to be considered in any patient becoming acutely ill and developing renal failure. Renal impairment: For patients with renal impairment, gabapentin, which is primarily excreted really and lacks active metabolites, requires dosage adjustment. Therefore, it is crucial to modify both the dose amount and frequency accordingly. After oral administration and absorption, gabapentin circulates essentially unbound to serum proteins. In addition, gabapentin does not undergo hepatic metabolism, unlike most other antiepileptic drugs, and is eliminated almost entirely by renal excretion with a clearance that approximates the glomerular filtration rate. The dependence on the kidney for gabapentin excretion can lead to toxicity in people with decreased kidney function.6,7 Manifestations of toxicity include dizziness, confusion, lethargy, myoclonus, ataxia, and tremulousness.6 The therapeutic range for gabapentin in blood is about 2 to 20 mg/mL (12-120 mol/L), m Pharmacokinetics and renal handling Challenges to achieving therapeutic concentrations necessary to achieve efficacy require consideration of the pharmacokinetic properties of both gabapentin and pregabalin. Metabolism: not metabolized Excretion: Renal Mechanism of Action: GABA analogue, but has no effect on GABA binding, uptake or degradation; mech for analgesic and anticonvulsant activity unknown See Also Seizure Anticonvulsants Anticonvulsant levels and reloading Neuropathy References ↑ Epocrates. Gabapentin Monograph. Gabapentin has a low oral bioavailability that is dose-dependent and a relatively short half-life. Together this results in a need for frequent daily dosing (generally three times daily). Gabapentin is predominately renally eliminated as unchanged drug, and therefore renal dosage adjustments are necessary in patients with renal insufficiency. Since gabapentin is almost exclusively eliminated by renal excretion, the larger treatment effect observed in patients ≥75 years may be a consequence of increased gabapentin exposure for a given dose that results from an age-related decrease in renal function. Pharmacokinetic properties are important to consider in evaluating the usefulness of new antiepileptic drugs (AEDs). Gabapentin is a new, water-soluble, antiepileptic agent with properties of an amino acid. This drug is rapidly absorbed and exhibits dose-dependent bioavailability as a result of a sa Excretion: Removed from your body by kidneys. If kidneys are slow, gabapentin stays longer, so dosing needs to be careful. 11. Patient Education What to Know: Take as Told: Always take gabapentin exactly as your doctor prescribed mg per day. Watch for Problems: If you feel very dizzy or confused, tell your doctor. The renal excretion of gabapentin involves a component of active secretion via an organic cation transporter (OCT2) in the kidney [11]. Other substrates for OCT2 include histamine, and guanidine derivatives such as creatinine and cimetidine [12]. Gabapentin is eliminated in urine unmetabolized at a rate proportional to creatinine clearance.24In patients with renal impairment, with unaltered gastrointestinal absorption, gabapentin half-life can be prolonged up to 132 hours (with-out dialysis),30 placing patients with chronic kidney disease at an increased risk for toxicity.
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 |  |
 |  |
 |  |
 |  |
 |  |