Gallery
Photos from events, contest for the best costume, videos from master classes.
 |  |
 | |
 | |
 |  |
 |  |
 |  |
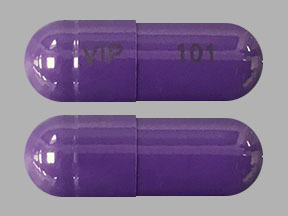
Amneal Pharmaceuticals of New York LLC: Gabapentin capsules are indicated for: Management of postherpetic neuralgia in adults. Adjunctive therapy in the treatment of partial onset seizures, with and without secondary Gabapentin may be administered as the oral solution, capsule, or tablet, or using combinations of these formulations. Dosages up to 50 mg/kg/day have been administered in a long-term clinical study. Do not take gabapentin oral solution if you are allergic to gabapentin or any of the other ingredients in gabapentin oral solution. See the end of this Medication Guide for a complete list of ingredients in gabapentin oral solution. n Oral Solution. See the end of this Medication Guide for a complete list of ingredients in Gabapent. n Oral Solution. What should I tell my healthcare provider before t. have diabetes • are pregnant or plan to. become pregnant. It is not known if gabapentin can harm . The NDC code 65162-698 is assigned by the FDA to the product Gabapentin which is a human prescription drug product labeled by Amneal Pharmaceuticals Llc. The product's dosage form is solution and is administered via oral form. The product is distributed in 2 packages with assigned NDC codes 65162-698-54 50 cup, unit-dose in 1 carton / 5 ml in 1 cup, unit-dose (65162-698-39), 65162-698-90 473 DESCRIPTION Gabapentin oral solution is supplied as an oral solution containing 250 mg/5 mL of gabapentin. The inactive ingredients for the oral solution are anise flavor, artificial strawberry flavor, glycerin, hydrochloric acid, purified water, sodium hydroxide and xylitol. Gabapentin oral solution contains 250 mg of gabapentin per 5 mL (50 mg per mL) and the following inactive ingredients: anise flavor, artificial strawberry flavor, glycerin, hydrochloric acid, purified water, sodium hydroxide and xylitol. Gabapentin oral solution contains 250 mg of gabapentin per 5 mL (50 mg per mL) and the following ingredients: acesulfame potassium, banana flavor NAT & ART, glycerin, methylparaben, propylparaben, propylene glycol, saccharin sodium and purified water. Oral solution: 250 mg per 5 mL (50 mg per mL), clear pale yellow to yellow solution. Gabapentin oral solution is contraindicated in patients who have demonstrated hypersensitivity to the drug or its ingredients. Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS), also known as multiorgan hypersensitivity, has occurred with gabapentin. Gabapentin oral solution is contraindicated in patients who have demonstrated hypersensitivity to the drug or its ingredients. Gabapentin is a Oral Solution in the Human Prescription Drug category. It is labeled and distributed by Amneal Pharmaceuticals Llc. The primary component is Gabapentin. Sample Package? 65162-698 National Drug Code registration, ingredients, and packaging details. Gabapentin oral solution is supplied as an oral solution containing 250 mg/5 mL of gabapentin. The inactive ingredients for the oral solution are anise flavor, artificial strawberry flavor What is NDC 65162-698-90? The NDC Packaged Code 65162-698-90 is assigned to a package of 473 ml in 1 bottle of Gabapentin, a human prescription drug labeled by Amneal Pharmaceuticals Llc. The product's dosage form is solution and is administered via oral form. Is NDC 65162-698 included in the NDC Directory? Gabapentin Oral Solution, 473 ml (Xylitol Free), RX GENERICS, Manufacturer Item #:65162069890, Patterson Item #:07-893-5342 Gabapentin oral solution contains 250 mg of gabapentin, USP per 5 mL (50 mg per mL) and the following inactive ingredients: acesulfame potassium, carboxymethylcellulose sodium, magnasweet, peppermint flavor, potassium sorbate and strawberry anise flavor. Gabapentin oral solution contains 250 mg of gabapentin, USP per 5 mL (50 mg per mL) and the following inactive ingredients: acesulfame potassium, carboxymethylcellulose sodium, magnasweet, peppermint flavor, potassium sorbate and strawberry anise flavor. The inactive ingredients for the oral solution are glycerin, xylitol, purified water and artificial cool strawberry anise flavor. Gabapentin is described as 1-(aminomethyl)cyclohexaneacetic acid Do not take Gabapentin Oral Solution if you are allergic to gabapentin or any of the other ingredients in Gabapentin Oral Solution. See the end of this Medication Guide for a complete list of ingredients in Gabapentin Oral Solution. The active ingredient in gabapentin oral solution is gabapentin, USP, which has the chemical name 1- (aminomethyl)cyclohexaneacetic acid. The molecular formula of gabapentin, USP is C 9 H 17 NO 2 and the molecular weight is 171.24. The active ingredient in gabapentin oral solution is gabapentin, USP, which has the chemical name 1- (aminomethyl)cyclohexaneacetic acid. The molecular formula of gabapentin, USP is C9H17NO2 and
Articles and news, personal stories, interviews with experts.
Photos from events, contest for the best costume, videos from master classes.
 |  |
 | |
 | |
 |  |
 |  |
 |  |